“Where do the molecules come from?” Connecting models and other resources to support explanation
Case at a Glance
Investigation: Conservation of Matter
Investigation Phase: Constructing Explanations
Teaching Practices:
Providing opportunities for students to make conceptual progress by making connections across different models
Introducing resources as tools for sensemaking
Positioning student contributions as important for community knowledge development
About the Classroom: This is a fifth-grade multilingual classroom where children speak either Spanish or Portuguese in addition to learning English. Their teacher, Lindsay, speaks some Spanish and is assisted by two instructional aides who provide language support.
Context: The case occurs within the fifth-grade Conservation of Matter Investigation, in a larger unit focused on structures and properties of matter. Students shared initial models of why a glass bottle filled with water breaks when the bottle is frozen, then investigated how the weight and volume of water changes when it freezes. They return to their initials models in light of a new question.
Introduction
Students in Lindsay's class are trying to explain a puzzle from their investigations: how can water get bigger but stay the same weight as it freezes? To help them develop explanations, Lindsay provides opportunities for students to move back and forth between their investigation results, pen-and-paper models, and a computer simulation. First, she facilitates a discussion about students' initial models of how water behaves when frozen. Students begin to wonder and disagree about whether the number of molecules changes and how molecules can spread. In light of these puzzles, the class revisits a computer simulation that helps them visualize molecules and develop explanations.
In this case, we see how having students make connections across models and other resources can support them to develop explanations as:
Students map their investigation results to their pen-and-paper models
Students pose new questions as they think about how to represent and explain findings in their pen-and-paper models
Students use a simulation to make progress on their questions
Case Background
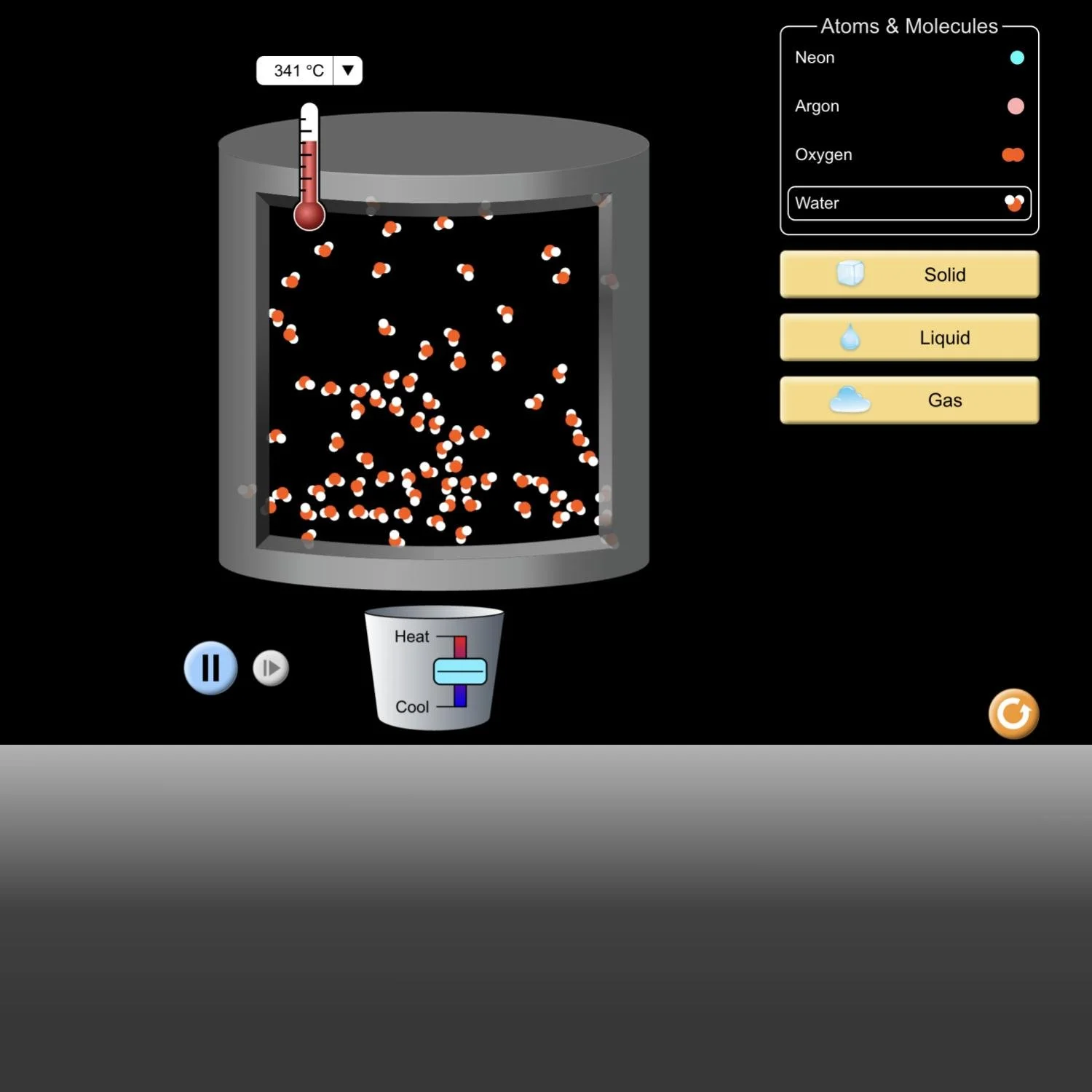
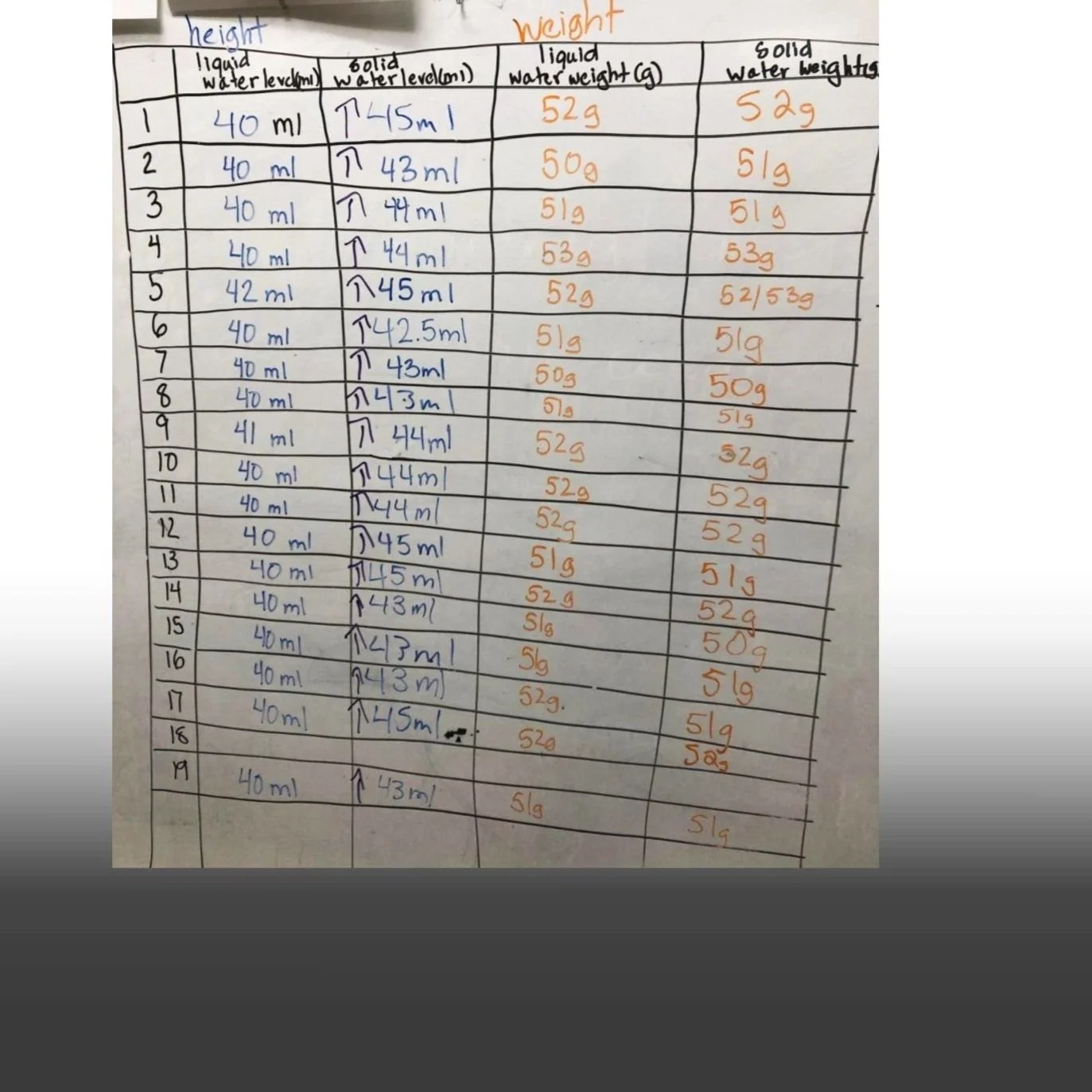
Students first watched a video that shows a glass bottle exploding when the water inside it freezes (Image 1). They drew pen-and-paper models that could explain the molecular changes that led to the explosion. To create their models, students built from prior classroom experiences, including engaging with a computer simulation (Image 2). Their models showed different ideas about what happened to the water in the glass bottle (Images 3 & 4), leading to further questions and investigation. After students investigated how water level and weight change as water freezes (Image 5), they considered the class data (Image 6) and agreed that as water freezes, it grows in volume but stays the same weight.
Uncertainty in Action
Students map investigation results to their pen-and-paper models
After working in small groups to discuss three students’ initial models, Lindsay facilitates a whole group discussion to evaluate the models in light of students' investigation findings. Rafael's model (Image 7) shows water molecules coming together as the temperature drops and explains that the bottle breaks because the cap freezes and air cannot escape.
Lindsay: Can this model explain how water gets bigger when it freezes? Rafael why do you say no? It's great this is your own model. It is okay that you think differently.
Rafael: Because I drew it– the first one bigger than the others.
Lindsay: So, Rafael, that's okay. You have new knowledge that you're using to apply to your thinking. It is great that you're changing what you're going to think about. So, Rafael notices that his molecules are bigger than his last ones. Can you say more about that, Rafael? (Lindsay points to the bottle in Rafael’s model, Image 8.)
Rafael: Because the first one I did the glass bottle bigger and the other ones I forgot to like do the same height of the glass bottle.
Lindsay: So, you're noticing that the bottle is different. Are you noticing anything that is different about the molecules inside? (Lindsay points to the molecules in Rafael’s model, Image 9.)
Rafael: Yeah. They're getting closer.
Lindsay: They're getting closer. Santiago, were you raising your hand?
Santiago: Yes, because I think that you were telling what is happening with the molecules on the first bottle, they are spread from the sides. And the second picture, they are getting closer. And on the third picture, the bottle explode and the molecules is all together. (Santiago shows the molecules exploding after coming together, Image 10.)
Lindsay: Okay, so, on the first one I believe you agree with Rafael. Molecules are spread out. And the second one, the molecules get closer. And then in the third one, they're very close together. So, we are going to give a yes or a no. Does this model explain how water gets bigger when it freezes?
Victoria: I don't know expanded but stay together.
Casandra: They get away or they stay together? Like they separate or stay together?
Victoria: Que muita pessoa falou que no segundo desenho elas estão separando e explodindo, só que como a gente pode ver no desenho lá eles se estão juntando. Então, a pergunta é como que eles explodiram e se juntaram...Então essa é a minha pergunta. [Yeah, because like a lot of people said in the second drawing that they are separating and exploding, but as we can see in the drawing there, they are coming together. So how are they exploding and then come together...so I’m asking.] (Victoria gestures the molecules coming together and exploding, Image 11.)
Francisco: I am going to try to explain what she says. She say… some peoples say the water is spread out and-but this explanation the water is close together.
Lindsay: Okay, so, some people are saying that the molecules are spread out, but this picture is showing them together.
As the discussion continues, students make sense of whether molecules that come together can cause water to expand. Rafael decides to revise his model to show the idea that the water level gets higher as the water freezes.
What we see…
-
After their investigation, students grapple with how to apply findings as they look to reconcile their initial models with their investigation results. This points them to a new question. How can water molecules be coming closer together while also making water bigger as it freezes?
-
Students draw on the investigation results to stabilize a shared understanding- that water increases in volume as it freezes.
Students' initial models serve as a base for further refinement of explanations.
Rafael and others draw on earlier work (including engagement with the simulation) for the idea that as substances get colder, their molecules come closer together.
-
Validating that thinking changes by doing science.
Making connections across modeling work by helping students see contradictions.
Inviting, highlighting and revoicing student ideas.
Students pose new questions as they consider how to represent and explain findings in their models
In contrast to Rafael's model, Sada's model (Image 12) shows water molecules pushing on the sides of the bottle as air fills the center. In trying to evaluate whether Sada's or Rafael's model is more correct, Victoria voices that she does not know the weight of the bottles in Sada's drawings and therefore cannot draw a conclusion.
Lindsay: So, Victoria is saying "I don't know the weight because it is a drawing.” Is there any way to know the weight by looking at the drawing?
Rodrigo: I think it [the weight in the models] stays the same and is heavier. Because like the molecules are light… The molecules in the first one like stay like, Como se diz mais leve? [How do you say lighter?]
Multiple students: Light
Lindsay: What do you mean that the molecules are light? What does that mean?
Multiple students raise their hands and speak.
Rodrigo: Because the first one has not- Como se diz muito menos [How do you say much less?]
Students: Not as much, not as many.
Rodrigo: Because the first one has not as much as the next one.
Lindsay: Okay, so, in this one (points to the first panel of the model, Image 13) the molecules are light because it doesn’t have too many. Okay, I am going to write that down. Rodrigo thinks the molecules are light because there aren’t that many. What do you think about that?
Rafael: One question I have is like how does the molecules–how does the last one have more molecules than the first one?
Audible agreement from other students; several hands raised.
Lindsay: So, that is a great question. Rafael, can you say more about why you're coming at that question? Why does this bottle (Image 14, points to third panel) why does it have more molecules than this bottle (Image 15, points to first panel), why is that a question for you?
Rafael: Because like that's like crazy to think about how does the molecules– how does when it turns to ice get more molecules, and where does the molecules come from?
-
As students work to develop an explanation, they try to identify mechanisms. Perhaps the water is getting bigger because the molecules are multiplying. But how would that work? Where would the new ones come from? And why wouldn’t more molecules weigh more?
-
Students draw from experiential knowledge that as things grow, they tend to weigh more, but they also suspect that molecules can't spontaneously generate.
-
Orienting to an uncertainty (the relationship of weight and number of molecules).
Revoicing students' ideas and asking children to respond to one another.
Lindsay reintroduces the simulation and connects it to students' questions
Lindsay: So, we came up with our own question yesterday, which is "how can molecules spread out and stay together?" But there are some other questions that have come up from trying to figure out what happens to the molecules when liquid water turns to solid water and the bottle explodes (Image 16). So, who can read the first question for me? Rodrigo?
Rodrigo: Do water molecules make more water molecules as liquid water becomes solid water as it freezes?
Lindsay: Yes. So, there is this idea about sons being made, about division happening, and we really want to look at today, trying to figure out if we think more molecules are being made when liquid water turns to solid water. I already see that some people have an idea and they're indicating with their hands yes or no. So today, when we look at the simulation, this is one of the focus questions that we are going to try and answer and use the information from the simulation to do it. This is our second question that we are answering on the paper. So if you think "yes" in your small groups today, there are two other follow-up questions that you could think about. So, if you think "yes, molecules are making more molecules," I’m curious, where are those molecules coming from? Do you have a question, Dr. Eve?
Eve: So the simulation can help you, right, provide evidence, can help you see if there are more water molecules as liquid water becomes solid water. Does anyone have an idea, on the simulation, what will you look at to see if there are more molecules? How would you know if there were more molecules or if there were not, if there were the same number of molecules? Anyone have an idea?
Santiago: Counting the number of molecules.
Eve: And then what would you compare? What would you count and compare?
Santiago: I would count first and liquid water for see how much have and when I put solid water I will see if has more.
Eve: So, you'd count it liquid and then you'd see as it turned to solid water, if it had more molecules as it turns to solid, if it has less molecules as it turns to solid. Is there another option? More molecules, less molecules--
Santiago: The same.
Eve: Maybe the same? What do other people think? Is that something you might try today as you look at the simulation?
Lindsay reviews how to use the simulation and goes over the student sheet, focusing on how students can use the simulation to answer their questions. (Video of the simulation below)
16. Students’ unanswered questions.
What we see …
-
Students are uncertain about the mechanisms explaining their findings. They prepare to use the simulation to make progress on two questions: (1) how the molecules might come together yet spread out and (2) whether molecules are multiplying as water freezes.
-
Lindsay and Eve help students put their learning from their models and prior experiences in conversation with the simulation to 1) generate questions and claims and 2) provide evidence for their changing thinking.
-
Reviewing questions and puzzles to establish a purpose.
Introducing a resource as a tool for sensemaking.
Inviting children to consider how to use a resource to make progress on their puzzles.
Students use the simulation to explore ideas related to how water molecules change with changes in temperature. Find this resource at https://phet.colorado.edu/sims/html/states-of-matter-basics/latest/states-of-matter-basics_en.html
Students make sense of the simulation together
After using the simulation and answering questions in writing (student sheet, Images 17 & 18), students discuss what they learned. The conversation focuses on what happens to the molecules as the temperature decreases and the liquid water turns solid.
Lindsay: How do you know when the liquid water is frozen in the simulation?
Rodrigo: Like start and stop. Like in the beginning the molecules like moving crazy, but the molecules like start stopping.
Bertrand: They start separating and making spaces to separate.
Lindsay: They start separating. So they started separating and making spaces? What do you mean by making spaces?
Bertrand: Start separating some parts... some parts have no molecules. (Bertrand draws his idea on the board, Image 19.)
Lindsay: Ahh, got it. So when they separated out, they created empty spaces where no molecules are. Okay, thank you. Thank you for showing me. So these are the spaces with no molecules (draws arrows pointing to the spaces, Image 20). At what temperature does liquid water becomes solid water? And how do we know? What else?
Bianca: They stay the same. And like freezes and get together.
Lindsay: Okay, so when liquid water becomes solid water, they get together? Any other ideas, Francisco?
Francisco: 0°C or 40°F because the molecule stay together.
Lindsay: The molecules stay together.
Francisco: And empty space.
Lindsay: So you think it’s 0°C. You think the molecules stay together, but you also agree that they are making space like what Bertrand says? Alright.
What we see …
-
In this episode, students have used the simulation to make progress toward reconciling the contradiction that drove their learning. While it is true that molecules come closer together as liquids solidify, upon freezing, water molecules come together in a way that creates regular spaces between them.
-
Students use the simulation to resolve the puzzle of how freezing water can both expand and have its molecules come together.
-
Inviting, revoicing, and working with children’s ideas.
Supporting students to use resources for sensemaking by connecting their learning from the simulation to the questions they have about how something can expand but not weigh more.
Reflection
This case shows how putting models and other resources in conversation with one another can support students to develop explanations. By choosing student models that surfaced a contradiction (water molecules come together when water freezes, but must also come apart to make the bottle explode), Lindsay opens space for students to feel puzzled and pose questions. These questions become the driving force of students’ subsequent explanation and work with the simulation. When teachers make space for puzzles to emerge and introduce new resources as tools for making progress on questions, children experience a sense of purpose and can use new tools for meaning-making.
We encourage you to reflect on your teaching practices in light of your learning from this case:
Where might there be opportunities in my practice to identify and elevate tensions in students’ thinking toward eliciting puzzlement for rich sensemaking and deepening explanations?
How can I use models and other resources as supports for students’ sensemaking conversations ?
When do I position resources as tools to help students resolve uncertainties rather than as ways to replace student ideas? Where can I imagine doing this more?